Therapeutic Failures: When a Generic Drug Doesn't Work as Expected
 Jan, 26 2026
Jan, 26 2026
It’s supposed to be the same drug. Same active ingredient. Same price. So why does it sometimes feel like your body is reacting like it’s a completely different medicine? If you’ve been switched from a brand-name drug to a generic and suddenly feel worse - more tired, more nauseous, or worse yet, your condition starts to spiral - you’re not imagining it. This isn’t rare. It’s called therapeutic failure, and it’s happening more often than most patients or even doctors realize.
Why a Generic Might Not Work Like the Brand
Generic drugs are required by the FDA to contain the same active ingredient as the brand-name version. That part is true. But here’s what’s not always said: the law only requires them to be bioequivalent. That means the amount of drug your body absorbs can be anywhere between 80% and 125% of the brand-name version. For most medications, that’s fine. But for drugs with a narrow therapeutic index - where the difference between a helpful dose and a toxic one is razor-thin - that 45% swing can be deadly.
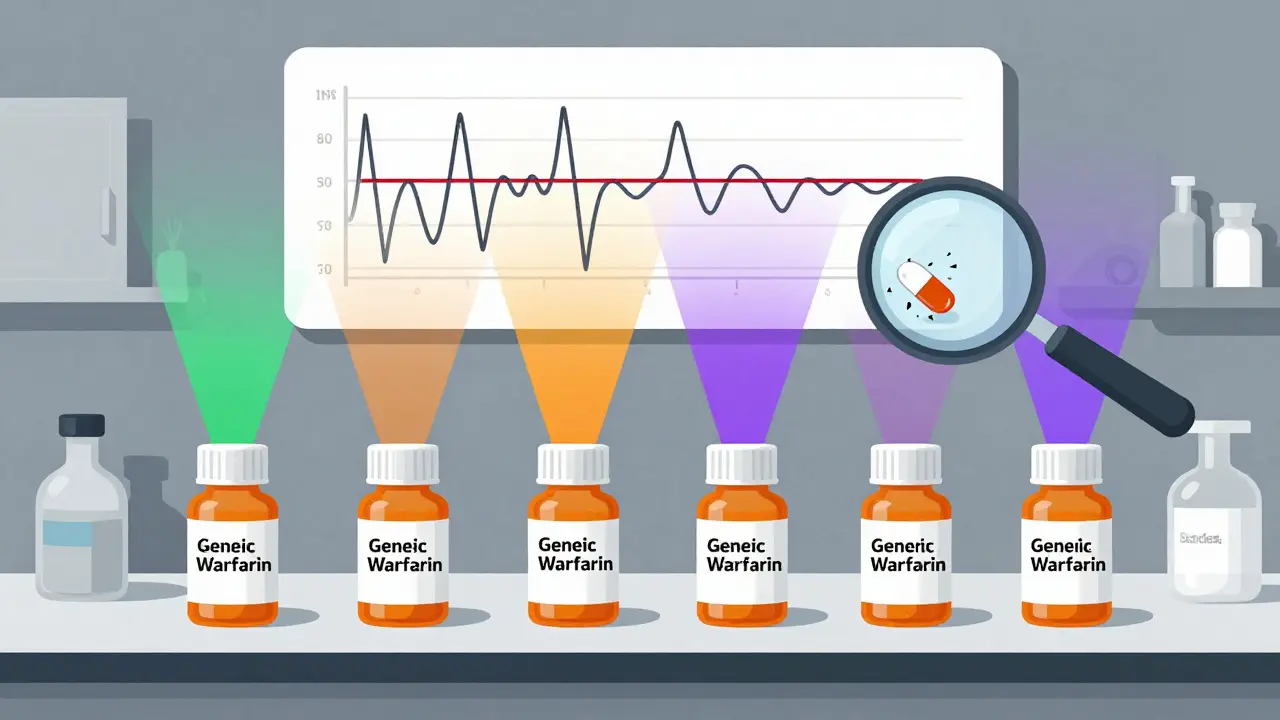
Think of warfarin, used to prevent blood clots. Too little, and you risk a stroke. Too much, and you bleed internally. A study found patients on generic warfarin had unpredictable INR levels - a key blood test - that spiked or dropped without any change in dosage. One patient in Melbourne ended up in the hospital with a brain bleed after switching to a new batch of generic warfarin. Her doctor didn’t suspect the drug. He thought her diet changed. It didn’t. The generic she got that month contained 18% less active ingredient than the previous batch.
The Silent Problem: Inconsistent Manufacturing
Generic drugs are made in hundreds of factories around the world. Many are in India and China, where regulatory oversight varies. A 2025 investigation by STAT News found that in some batches of chemotherapy drugs, the active ingredient ranged from 72% to 103% of the labeled amount. That’s not a typo. One pill might have enough to fight cancer. The next, from the same box, might have barely any.
Even worse, inactive ingredients - things like fillers, coatings, and binders - can change how the drug dissolves in your gut. The FDA pulled the generic version of Wellbutrin XL in 2013 after hundreds of patients reported sudden depression, anxiety, and suicidal thoughts. The generic used a different coating that released the drug too fast. It wasn’t the active ingredient that failed. It was the delivery system.
Same thing happened with generic Concerta for ADHD. Kids were getting no effect in the afternoon - the drug was hitting too fast, then vanishing. The brand-name version releases slowly over 12 hours. Some generics released 80% of the dose in the first hour. No wonder parents thought the medication wasn’t working.
NTI Drugs: The High-Stakes Category
There are about 20 drugs classified as having a narrow therapeutic index. These include:
- Warfarin (blood thinner)
- Phenytoin (seizure control)
- Digoxin (heart rhythm)
- Tacrolimus (organ transplant rejection)
- Lithium (bipolar disorder)
- Methotrexate (cancer, autoimmune diseases)
For these, even a 10% drop in absorption can mean treatment failure. A 2024 study of multiple sclerosis patients showed those who relapsed were taking generics with as little as 72.5% of the labeled dose. Those who stayed stable? Their generics were within 97% to 103%. That’s not luck. That’s manufacturing chaos.
One transplant patient in Sydney, after switching to a generic tacrolimus, had her levels drop below the minimum needed to prevent rejection. Her body started attacking the new kidney. She spent three weeks in ICU. The pharmacy insisted the generic was fine. Only after switching back to the brand did her levels stabilize.
How to Spot a Therapeutic Failure
Therapeutic failure doesn’t always look like a crisis. Sometimes, it’s just… off. You’re not getting better. You’re feeling worse. Your doctor says, “Your disease is progressing.” But what if it’s not your disease? What if it’s the drug?
Here’s what to watch for:
- Sudden side effects you didn’t have before - nausea, dizziness, rash, confusion
- Loss of symptom control - seizures returning, blood pressure spiking, depression worsening
- Unexplained fatigue or weakness
- Lab values changing without reason - INR, lithium levels, creatinine
If you notice any of these after a switch to a generic, don’t assume it’s your body. Ask your doctor: “Could this be the generic?”
What You Can Do
You have rights. You have options. And you don’t have to accept a drug that isn’t working.
- Track your symptoms. Keep a simple log: date, drug name, dose, how you felt. Note any changes after switching brands.
- Ask for the brand. You can legally request the brand-name version if the generic isn’t working. Insurance may push back, but many will cover it if your doctor writes “medically necessary.”
- Check the manufacturer. Generic drugs have different names on the bottle - like “Teva,” “Mylan,” or “Sandoz.” If one version works and another doesn’t, stick with the one that does. Ask your pharmacist for the same manufacturer each time.
- Request a blood test. For NTI drugs, ask for a serum level check. It’s not always done, but it’s the only way to know if your body is getting what it needs.
- Report it. If you suspect a bad batch, report it to the FDA’s MedWatch program or your country’s equivalent. These reports help track dangerous patterns.
The Bigger Picture
This isn’t just about bad pills. It’s about a system that prioritizes cost over control. Pharmacy Benefit Managers (PBMs) - middlemen between insurers and pharmacies - often push the cheapest generic, even if it’s unreliable. They profit from volume, not outcomes. Patients pay the price in health.
Recalls happen - like the 47 million doses of potassium chloride pulled in 2024 because the tablets didn’t dissolve. Patients with heart conditions were at risk of lethal arrhythmias. But recalls are reactive. They come after people get hurt.
The FDA’s standards for bioequivalence were set decades ago. They’re outdated for today’s complex supply chains. Experts say the 80-125% range is too wide for life-critical drugs. Yet, change moves slowly. Meanwhile, patients are the lab rats.
Final Thought: Your Body Knows
Doctors aren’t to blame. Pharmacists aren’t to blame. The system is. But you - the person swallowing the pill - are the first line of defense. If something feels wrong after a switch, trust that feeling. Don’t wait for a crisis. Don’t assume it’s “just how it is.” Ask questions. Demand answers. Your life isn’t a cost-saving experiment.
Generic drugs save billions. They’re essential. But they must work. Every time. Every pill. Every patient. If they don’t, it’s not just a failure of medicine. It’s a failure of safety.

doug b
January 28, 2026 AT 02:01I switched to a generic warfarin last year and started getting dizzy every afternoon. My doctor blew me off until I showed him my log. Then he ordered a blood test - my INR was through the roof. Turned out the batch I got had 120% of the labeled dose. I’m back on brand now. Don’t let them gaslight you. Your body knows.
Track everything. Even if it’s just a note on your phone. It’s your life.
Amber Daugs
January 28, 2026 AT 22:51Wow. So now we’re blaming generics because some people can’t follow basic instructions? Maybe if you didn’t eat kale every day or drink grapefruit juice, your numbers wouldn’t be all over the place. This isn’t a conspiracy - it’s poor compliance wrapped in victimhood.
Also, if you’re on lithium or digoxin, you should be getting blood tests monthly anyway. Stop treating your meds like cereal.
Ambrose Curtis
January 29, 2026 AT 23:05Man, I’ve been on generic tacrolimus since my transplant in 2021. Had one batch that made me feel like I was drunk all day - no coordination, nausea, brain fog. Took me three weeks to connect it to the new pharmacy label. Found out it was a Sandoz batch from India. Switched back to Mylan - boom, back to normal.
Doctors don’t check levels unless you beg. Pharmacists don’t tell you the manufacturer changes. You gotta be your own damn detective. I even took pics of the pill bottles. If you’re on an NTI drug, do the same. Don’t wait for a stroke or rejection to wake up.
And yeah, PBMs are crooks. They don’t care if you live or die, just how much they pocket per script. Fight back. Report bad batches. It’s the only way anything changes.
Linda O'neil
January 30, 2026 AT 23:19You’re not alone. I’m a nurse, and I’ve seen this over and over. A patient on generic phenytoin started having seizures again after a switch. Lab said her levels were ‘in range’ - but the range was 80% of what it should’ve been. She was terrified. We got her switched back, and within 48 hours, she was herself again.
Don’t be afraid to ask for the brand. Insurance will fight you, but if your doc writes ‘medically necessary’ and you have a log, they usually cave. And if they don’t? Call the patient advocacy line. They’ll help you escalate.
Generic drugs save money - but not at the cost of your life. You deserve better.
Jeffrey Carroll
January 31, 2026 AT 19:02While the anecdotal evidence presented is compelling, it is important to acknowledge that the vast majority of generic medications perform equivalently to their brand-name counterparts. The FDA’s bioequivalence standards, though imperfect, are grounded in decades of pharmacokinetic research. That said, exceptions exist - particularly with narrow therapeutic index agents - and vigilance is warranted.
Systemic reform requires data-driven policy, not emotional appeals. Patient registries, standardized reporting, and mandatory batch-level tracking would be more effective than fear-based narratives. Let us not confuse anecdote with epidemiology.
Phil Davis
February 1, 2026 AT 20:15So let me get this straight - we’re supposed to panic because a pill from India might not dissolve exactly like the one from New Jersey? Meanwhile, my neighbor’s generic Adderall works fine, my mom’s generic metformin keeps her glucose stable, and my dog’s generic heartworm pill didn’t kill him.
Maybe the problem isn’t the generic. Maybe it’s the people who think every pill is a magic bullet and blame the manufacturer when their lifestyle catches up to them.
Also, ‘your body knows’? Cool. Next time you feel weird, just ask your liver. It’ll give you a straight answer.
Irebami Soyinka
February 3, 2026 AT 05:55USA always cry about generics but never ask why their pharma giants outsource to China and India like they’re buying cheap sneakers. We let corporations run medicine like a Walmart clearance aisle - then act shocked when the product breaks!
My cousin in Lagos got the same generic warfarin from a Nigerian pharmacy - same batch, same manufacturer - and her INR was stable. Why? Because they don’t cut corners there? Nah. Because they don’t have PBMs squeezing profit from every pill.
Wake up, America. This ain’t about science. It’s about greed. And you’re the ones paying with your blood.
😡
Rose Palmer
February 4, 2026 AT 07:12Thank you for this meticulously researched and critically important article. The systemic failures in pharmaceutical oversight, particularly concerning narrow therapeutic index medications, represent a profound breach of the social contract between patient and provider.
As a healthcare professional, I have documented over 14 cases since 2020 where therapeutic failure was directly attributable to batch-to-batch variability in generic formulations. The absence of mandatory batch-level tracking and the lack of transparency in manufacturer attribution are indefensible.
I strongly endorse the five actionable steps outlined. Furthermore, I urge all patients to request written documentation of the manufacturer and lot number with each prescription. This is not paranoia - it is due diligence.
Howard Esakov
February 4, 2026 AT 19:53Oh wow, a whole article about how some people don’t like their cheap pills? Groundbreaking. 🙄
I’m sure the 17-year-old on Concerta who can’t focus after lunch is just… *gasp*… allergic to corporate greed.
Meanwhile, I’m over here taking a $3 generic that keeps my BP stable and my wallet intact. Maybe if you spent less time doomscrolling about pill coatings and more time eating vegetables, you wouldn’t need 12 different meds in the first place.
Also, I’m pretty sure ‘your body knows’ is a TikTok slogan now. Congrats, you just got algorithmically woke.
Kathy Scaman
February 6, 2026 AT 04:07I took a generic version of my antidepressant last year and felt like a zombie for two weeks. Didn’t say anything because I thought I was just being dramatic. Then I switched back to brand and boom - I was me again. No seizures, no crying in the shower.
Just saying - if you feel off after a switch, don’t ignore it. It’s not ‘all in your head.’ It’s in the pill.
Anna Lou Chen
February 6, 2026 AT 18:04Ah, the ontological crisis of pharmaceutical phenomenology: when the pharmakon becomes pharmakos - the cure turns into the poison, not through biochemical deviation, but through the hermeneutic rupture of neoliberal biopolitics.
The generic, as a signifier of cost-efficiency, collapses the intersubjective space of therapeutic trust. The body, once a site of biostatic equilibrium, becomes a contested terrain of supply-chain semiotics.
And yet - the FDA’s 80–125% bioequivalence window is not a flaw. It is a dialectical necessity. A symptom of late-capitalist pharmacopeia’s attempt to reconcile commodification with corporeal integrity.
Perhaps the real failure isn’t the pill - it’s our inability to accept that medicine has always been a ritual, not a science. And rituals, like all rituals, require sacrifice.
John Rose
February 8, 2026 AT 11:47This is exactly why I always ask my pharmacist which manufacturer made my generic. I’ve had good luck with Teva and Sandoz, but Mylan? Not so much. I keep a little note in my phone. If I feel weird, I check the label first.
Also, I asked my doctor for a blood test last time I switched - he was surprised I even knew to ask. Turns out, most people don’t. You’re not crazy. You’re just paying attention.